
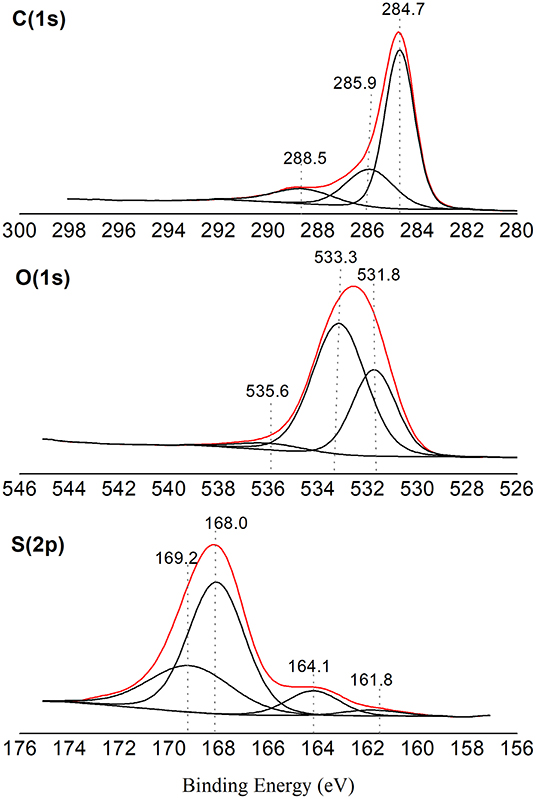
Introduction Several industrial applications find their origin in the existence of ion diffusion in glasses. The present results evidence an important influence of the nature of the modifier cation for silver glasses more covalent M–S bonds, less negative sulfur atoms and a more homogeneous electronic distribution on sulfur atoms are observed as compared to sodium glasses. On the whole it was shown that the modifier cation induces important electronic redistribution in the glassy matrix which extends to all sulfide atoms, either bridging or non-bridging, the evolution being all the more important that the modifier content is high. The study has been focused on the influence of the content and nature of the modifier. The structure and electronic structure of a series of thiogermanate glasses has been investigated by means of X-ray photoelectron spectroscopy ( XPS) and ab initio calculations with optimisation of geometrical parameters.


 0 kommentar(er)
0 kommentar(er)
